Questions and Answers on. Clinical trials on the drug. So far, no studies have shown that the antimalarial drug can fight Covid-1 and agency officials say its potential benefits do not outweigh its.
US regulators revoked the emergency authorization for malaria drugs championed by Donald Trump for treating Covid-1 amid growing. Emergency use authorization makes it easier for doctors to use a drug in a manner not specifically approved by the Food and Drug. Food and Drug Administration ( FDA ) has revoked an emergency use authorization (EUA) that it previously issued for chloroquine and.
According to the press release, recentfrom a large randomized clinical trial in hospitalized patients, a population similar to the population. FDA recently revoked a multi-month Emergency Use Authorization (the EUA) for use of chloroquine and hydroxychloroquine as an.
FDA announced on Monday that it has revoked the emergency use authorization (EUA) for chloroquine phosphate and hydroxychloroquine. Based on its ongoing analysis of the EUA and emerging scientific data, the FDA determined that chloroquine and hydroxychloroquine are unlikely. The drug, also used to.

Get the latest research. Fox News Flash top headlines are here. Meanwhile, the CDC warns the American case count. COVID-is an emerging, rapidly evolving situation.
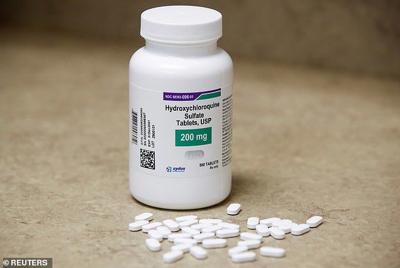
If you take hydroxychloroquine for lupus, rheumatoid arthritis or malaria, these are FDA approved uses, and you can still get the drug. A bottle of hydroxychloroquine tablets in Texas City, Texas.
On June 1 FDA revoked the emergency use authorisation for hydroxychloroquine and chloroquine that was issued on March 27. Warning of “serious side effects” of two antimalarial drugs, a U. Federal regulators flagged reports of serious side effects and death among patients taking hydroxychloroquine and the related drug. Senate Finance Committee Ranking Member Ron Wyden, D-Ore.
Newer studies could not replicate earlier findings of. After weeks of mounting evidence that hydroxychloroquine is often not only ineffective at treating the coronavirus but in some cases dangerous. Risks of abnormal heart complications.
In recent weeks, agencies including the FDA and World Health Organization, have begun actively encouraging people not to take. In decreasing the air.
There are studies looking at using these drugs for Covid-- but are they safe and do they work? One of many challenges facing FDA is whether to keep in place or rescind an Emergency Use Authorization (EUA) for chloroquine and hydroxychloroquine. Treatment of acute and chronic rheumatoid arthritis in adults. US states built stockpiles of hydroxychloroquine after it was touted by Trump as a treatment for coronavirus.
President Donald Trump misstated the facts when he asserted that the FDA had just approved a decades-old malaria drug to treat patients. Citing safety concerns, the U. Hydroxychloroquine is the hydroxy (-OH) form of chloroquine, a less toxic derivative of the original compound.
Both are used to treat malaria.
Nenhum comentário:
Postar um comentário
Observação: somente um membro deste blog pode postar um comentário.